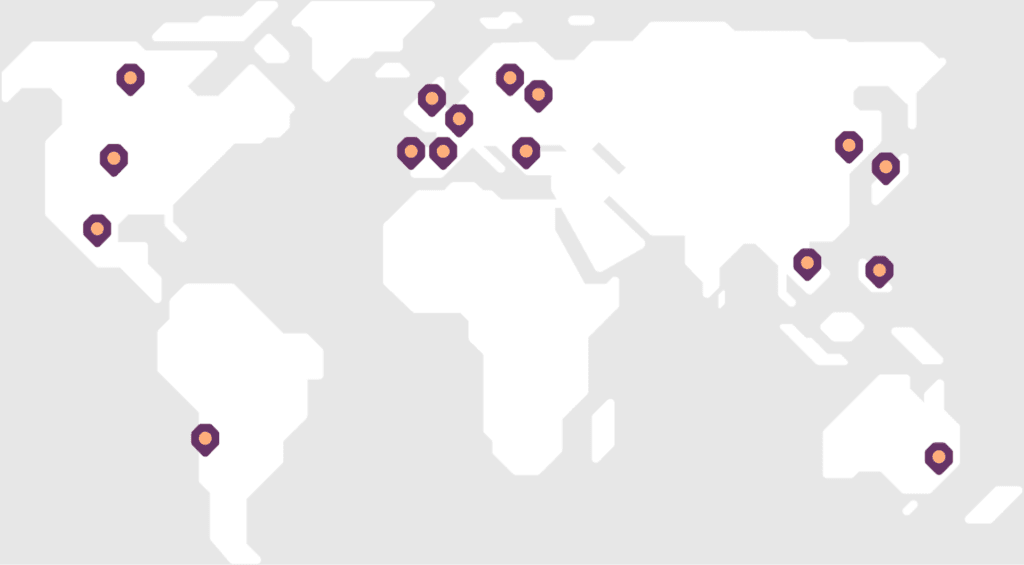
GCPre Consulting’s mission is to expand the knowledge and understanding of GCP across all stakeholders in clinical research and to facilitate its implementation in alignment with international standards and regulatory expectations.

Registered Quality Assurance Professional
Since 2023
Since 2022
Clinical Research and Clinical QA Consultant
GCPre Consulting is an initiative started by Marisol Diaz in 2024 by combining experience and passion.
Marisol has worked in life sciences across the globe since 2005. Throughout her career, she has taken on diverse roles, collaborating with clinical research sites, developing processes for clinical site management and conducting audits among many others.
While building on this experience, she developed a strong passion for first time quality and a commitment to helping others to understand and implement it.
Thanks to her diverse expertise across different functions in both CRO and pharmaceutical company, enriched by her global exposure, Marisol was able to build a unique skill set that enables her to offer custom-made solutions for clinical research.
Marisol holds a Bachelor of Science in Pharmacy, and two Postgraduate Diplomas in Clinical Trials Monitoring and in Drug Registration and Regulatory Affairs. She is a Registered Quality Assurance Professional for GCP and an active member of the Research Quality Organization, the Society of Quality Assurance, the Association of Clinical Research Professionals and the Spanish Association for Quality.